
Selenide-Catalyzed Stereoselective Construction of Tetrasubstituted Trifluoromethylthiolated Alkenes with Alkynes
Congratulations! The first publication of our group in Chem. Eur. J , "Selenide-Catalyzed Stereoselective Construction of Tetrasubstituted Trifluoromethylthiolated Alkenes with Alkynes" has been accepted recently (Jin-Ji Wu, Jia Xu and Xiaodan Zhao* Chem. Eur. J . 2016, 22, 15265–15269).

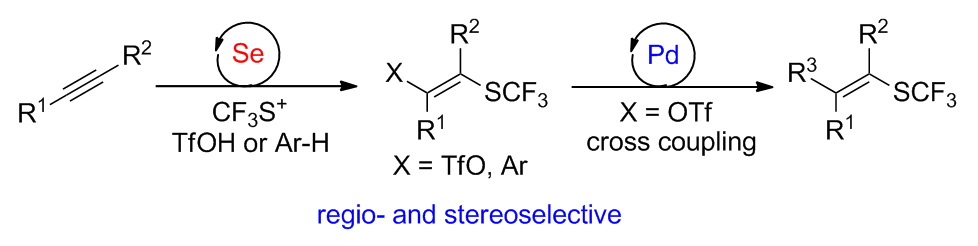
The efficient regio- and stereoselective construction of tetrasubstituted alkenes is challenging and very important. For this purpose, we have developed an efficient approach to synthesize tetrasubstituted trifluoromethylthiolated alkenes from simple alkynes in excellent regio- and stereoselectivities by selenide-catalyzed multicomponent coupling. Using this method, trifluoromethylthiolated alkenyl triflates and arenes were achieved. In particular, the triflates could be further converted into carbofunctionalized alkenes by palladium-catalyzed cross coupling reactions. Our method provides a new pathway for the construction of trifluoromethylthiolated tricarboalkenes. This work presents the first example of selenide-catalyzed trifluoromethylthiolation of alkynes and enables the challenging functionalizations of alkynes.
Previous page: Assist. Prof. Yu Zhao Visited Our Group
Next page: The 6th Lingnan Organic Chemistry Forum
