
Combination of Lewis Basic Selenium Catalysis and Redox Selenium Chemistry: Synthesis of Trifluoromethylthiolated Tertiary Alcohols with Alkenes
Congratulations! The publication of our group in Org. Lett., "Combination of Lewis Basic Selenium Catalysis and Redox Selenium Chemistry: Synthesis of Trifluoromethylthiolated Tertiary Alcohols with Alkenes" has been published recently (Zechen Zhu, Jie Luo, and Xiaodan Zhao* Org. Lett. 2017, 19, 4940–4943.).

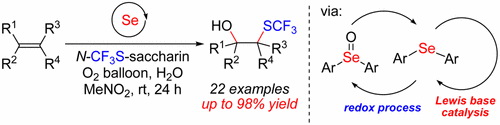
A new and efficient method for diaryl selenide catalyzed vicinal CF3S hydroxylation of 1,1-multisubstitued alkenes has been developed. Various trifluoromethylthiolated tertiary alcohols could be readily synthesized under mild conditions. This method is also effective for the intramolecular cyclization of alkenes tethered by carboxylic acid, hydroxy, sulfamide, or ester groups and is associated with the introduction of a CF3S group. Mechanistic studies have revealed that the pathway involves a redox cycle between Se(II) and Se(IV) and Lewis basic selenium catalysis.
Previous page: Chiral Selenide-Catalyzed Enantioselective Construction of Saturated Trifluoromethylthiolated Azaheterocycles
Next page: The 7th Lingnan Organic Chemistry Forum
