
Selenide-catalyzed enantioselective synthesis of trifluoromethylthiolated tetrahydronaphthalenes by merging desymmetrization and trifluoromethylthiolation
Congratulations! The publication of our group in Nature Commun., "Selenide-catalyzed enantioselective synthesis of trifluoromethylthiolated tetrahydronaphthalenes by merging desymmetrization and trifluoromethylthiolation" has been published recently (Jie Luo, Qingxiang Cao, Xiaohui Cao*, and Xiaodan Zhao* Nat. Commum., 2018, 9, 527 ).

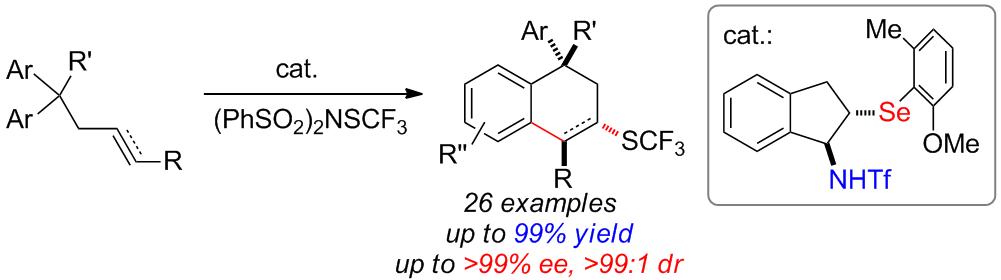
Trifluoromethylthiolated molecules are an important class of biologically active compounds and potential drug candidates. Because of the lack of efficient synthetic methods, catalytic enantioselective construction of these molecules is rare and remains a challenge. To expand this field, we herein disclose a bifunctional selenide-catalyzed approach for the synthesis of various chiral trifluoromethylthiolated tetrahydronaphthalenes bearing an all-carbon quaternary stereocenter with gem-diaryl-tethered alkenes and alkynes by merging desymmetrization and trifluoromethylthiolation strategy. The products are obtained in high yields with excellent enantio- and diastereo-selectivities. This method can be applied to the desymmetrization and sulfenylation of diols as well. Computational studies reveal that selenide can activate the electrophilic reagent better than sulfide, confirming the higher efficiency of selenide catalysis in these reactions. On the basis of the theoretical calculations, an acid-derived anion-binding interaction is suggested to exist in the whole pathway and accounts for the observed high selectivities.
Previous page: Catalysts for a more sustainable chemistry by Rhett Kempe
Next page: Green Catalysis and Sustainable Development Ruthenium a Key Actor by Pierre H. Dixneuf
