
Selenium-π-Acid Catalyzed Oxidative Functionalization of Alkynes: Facile Access to Ynones and Multisubstituted Oxazoles
Congratulations! The publication of our group in ACS Catalysis, "Selenium-π-Acid Catalyzed Oxidative Functionalization of Alkynes: Facile Access to Ynones and Multisubstituted Oxazoles" has been published recently (Lihao Liao, Hang Zhang, and Xiaodan Zhao*ACS Catal. 2018, 8, 6745−6750).

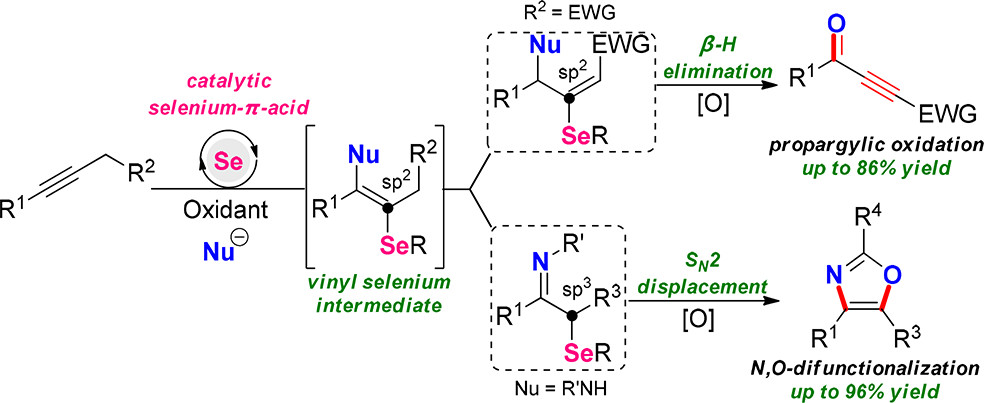
Unprecedented selenium-catalyzed propargylic oxidation of alkynes is disclosed. Various propargylphosphonates and 3-alkynoates were efficiently converted to valuable ynones via unusual C–C triple bond migration and deselenenylation at a vinyl carbon. By the strategies of tautomerization of enamine intermediate and SN2 displacement, similar conditions were effective for the oxidative difunctionalization of ynamides to afford multisubstituted oxazoles with high regioselectivity. Mechanistic studies revealed these detailed processes.
Previous page: Chiral Selenide-Catalyzed Enantioselective Allylic Reaction and Intermolecular Difunctionalization of Alkenes: Efficient Construction of C-SCF3 Stereogenic Molecules
