
Access to Saturated Thiocyano-Containing Azaheterocycles via Selenide-Catalyzed Regio- and Stereoselective Thiocyanoaminocyclization of Alkenes
Congratulations! The publication of our group in Organic Letters, "Access to Saturated Thiocyano-Containing Azaheterocycles via Selenide-Catalyzed Regio- and Stereoselective Thiocyanoaminocyclization of Alkenes" has been published recently (Wei Wei, Lihao Liao, Tian Qin, and Xiaodan Zhao* Org. Lett., 2019, 21, 7846-7850).

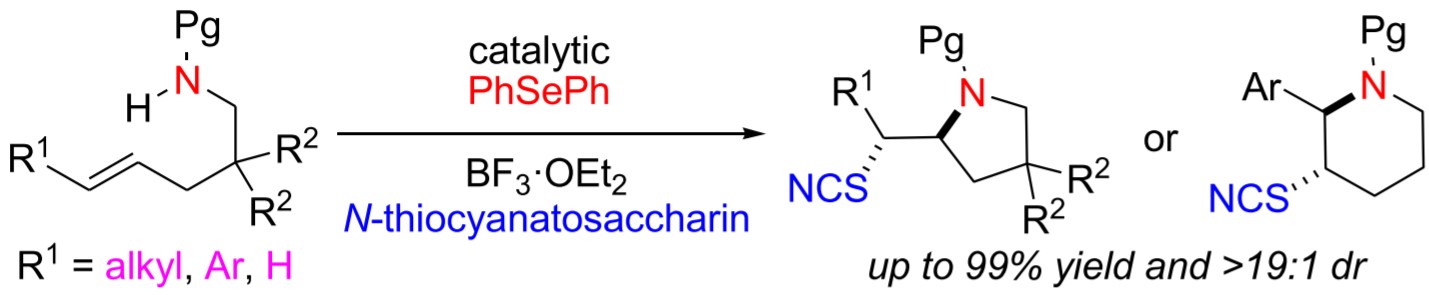
An efficient route for the synthesis of saturated thiocyano-containing azaheterocycles by selenide-catalyzed regio- and stereoselective thiocyanoaminocyclization of alkenes is disclosed. The desired products were obtained in moderate to high yields under mild conditions. The generality of this method was elucidated by its efficient application in thiocyano oxycyclization of alkenes.
Previous page: Enantioselective Azidothiolation and Oxythiolation of N-Allyl Sulfonamides and Organoselenium-Catalyzed Aza-Wacker Reactions
Next page: Enantioselective Construction of Axially Chiral Amino Sulfide Vinyl Arenes by Chiral Sulfide‐Catalyzed Electrophilic Carbothiolation of Alkynes
