
Chalcogenide-Catalyzed Intermolecular Electrophilic Thio- and Halofunctionalization of gem-Difluoroalkenes: Construction of Diverse Difluoroalkyl Sulfides and Halides
Congratulations! The publication of our group in Org. Lett., "Chalcogenide-Catalyzed Intermolecular Electrophilic Thio- and Halofunctionalization of gem-Difluoroalkenes: Construction of Diverse Difluoroalkyl Sulfides and Halides" has been published recently (Quanbin Jiang, Yaoyu Liang, Yuanyuan Zhang, and Xiaodan Zhao* Org. Lett., 2020, 22, 7581-7587).

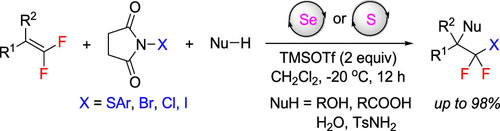
Thio- and halodifluoromethylated compounds are an important class of compounds in medicinal chemistry and organic synthesis. Herein, we report a facile method for the construction of these compounds via chalcogenide-catalyzed intermolecular electrophilic thio- and halofunctionalization of gem-difluoroalkenes. Simple treatment of gem-difluoroalkenes with electrophilic sulfur/halogen reagents and various O- or N-nucleophiles affords diverse multifunctionalized thio- and halodifluoromethylated compounds. This reaction features a relatively broad substrate scope, good functional group tolerance, and mild reaction conditions.
Previous page: Progress in the Synthesis of Aza-Heterocyclic Compounds via Selenium-π-Acid Catalysis
Next page: Selenopyrylium and Benzoselenopyrylium Salts (Uptate 2020)
