
Chiral Bifunctional Chalcogenide-Catalyzed Enantioselective Electrophilic Thiofunctionalization of Alkenes
Congratulations! The publication of our group in Chin. J. Org. Chem., "Chiral Bifunctional Chalcogenide-Catalyzed Enantioselective Electrophilic Thiofunctionalization of Alkenes" has been published recently (Quanbin Jiang, Xiaodan Zhao* Chin. J. Org. Chem., 2021, 41, 443-454).

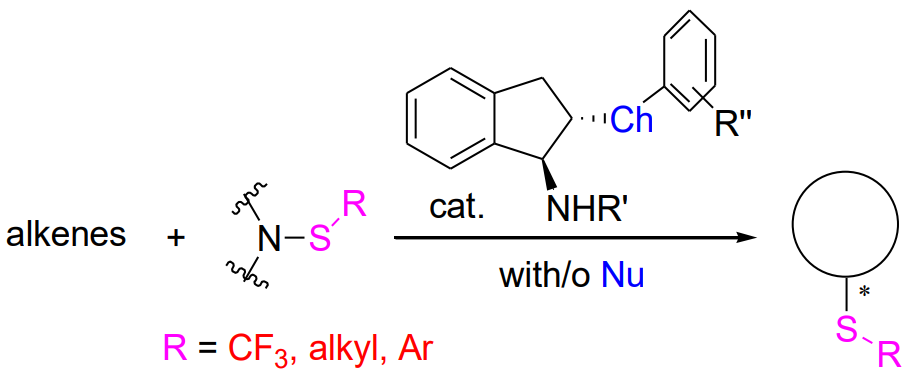
Chiral organosulfur compounds have a wide range of applications in the fields of medicinal chemistry and asymmetric synthesis. The development of new methods for the preparation of these compounds is an important task in organic synthetic chemistry. Enantioselective electrophilic thiolation of alkenes has emerged as a straightforward pathway for the synthesis of chiral sulfides. By this fashion, both the thio group and another valuable functional group can be introduced simultaneously into the parent alkene molecules. We designed and synthesized a series of chiral bifunctional chalcogenide catalysts and successfully applied them to intra- and intermolecular enantioselective trifluoromethylthiolation, alkylthiolation, arylthiolation of different kinds of alkenes. A variety of chiral sulfides were obtained with high enantioselectivities. This account summarizes the recent advances in chiral bifunctional chalcogenide catalyzed enantioselective thiofunctionalization of alkenes developed by our group, and the prospect of this field is also discussed.
Previous page: Efficient Synthesis of P-Chirogenic Compounds Enabled by Chiral Selenide-Catalyzed Enantioselective Electrophilic Aromatic Halogenation
