
Enantioselective Trifluoromethylthiolating Lactonization Catalyzed by an Indane-Based Chiral Sulfide
Congratulations! The first publication of our group in Angew. Chem. Int. Ed, "Enantioselective Trifluoromethylthiolating Lactonization Catalyzed by an Indane-Based Chiral Sulfide" has been published recently (Xiang Liu, Rui An, Xuelin Zhang, Jie Luo, and Xiaodan Zhao* Angew. Chem. Int. Ed. 2016, 55, 5846–5850).

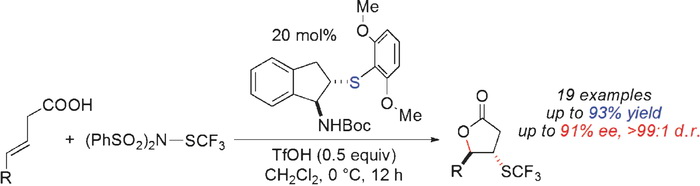
An efficient approach for enantioselective trifluoromethylthiolating lactonization entails the use of an indane-based bifunctional chiral sulfide catalyst and a new shelf-stable electrophilic SCF3 reagent. This transformation represents the first enantioselective trifluoromethylthiolation that is enabled by a catalyst with a Lewis basic sulfur center.
This research was rated as the inside back cover.
Previous page: Organoselenium-Catalyzed Synthesis of Oxygen- and Nitrogen-Containing Heterocycles
Next page: Prof. Zucai Suo Visited Our Group

